New EU Project “SafePolyMed”: Towards Safer Drug Treatment and Enhanced Patient Empowerment
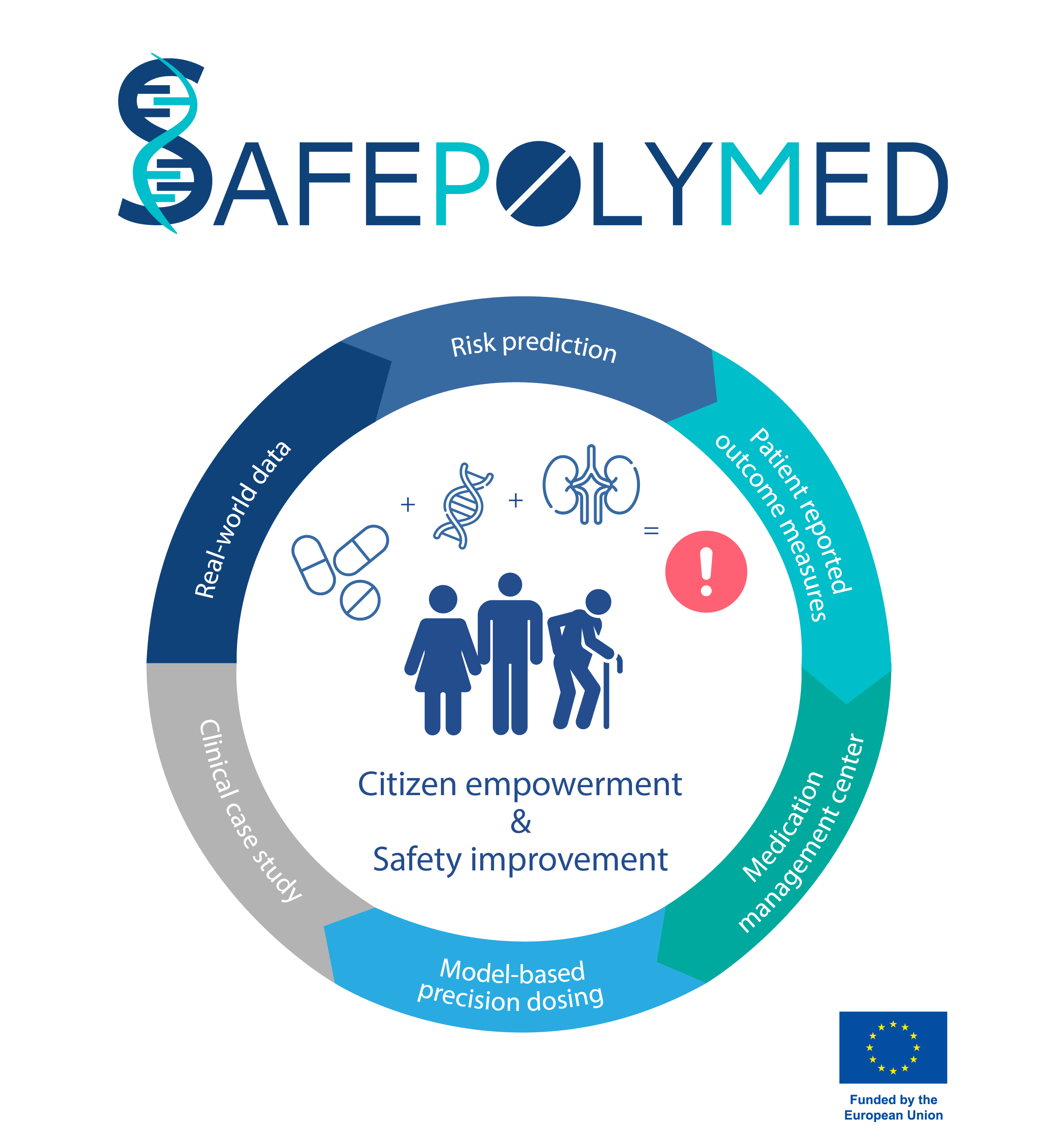
Adverse drug reactions (ADRs) are a major burden to our healthcare and economic systems. In Europe alone, approximately 197,000 deaths per year can be attributed to ADRs according to an assessment by the European Commission. The regular use of five or more medications concomitantly (polypharmacy), the coexistence of two or more long-term medical conditions or diseases (comorbidity) and genetic diversity have a major effect on drug efficacy and consequently, raise the incidence and severity of ADRs. Despite the fact that drug-drug interactions (DDIs) and drug-gene interactions (DGIs) are highly interconnected, in clinical practice, they are still considered separate entities. Hence, a more holistic approach taking into account individual disease states and drug-drug-gene interactions (DDGIs) is needed.
Aiming to increase overall patient safety, the new research project “SafePolyMed – Improving Safety in Polymedication by Managing Drug-Drug-Gene Interactions” seeks to develop innovative tools to define, assess and manage DDGIs for physicians and individual patients promoting enhanced citizen education and empowerment. Bringing together eleven partner institutions from across Europe, SafePolyMed receives a total funding of 5.6 Mio Euro under the European Union’s “Horizon Europe” Framework Programme for Research and Innovation.