New Generation of Advanced Cell-Based Medicine for Personalized Immunotherapy of Castration-Resistant Prostate Cancer
Researchers at the University of Ljubljana - Faculty of Medicine, University Medical Center, Celica Biomedical and the Blood Transfusion Center of the Republic of Slovenia have developed and clinically tested a new generation of autologous cell-based medicine for prostate cancer. The results showed that the survival of prostate cancer patients treated with autologous immunohybridomas (aHyC) prepared by fusion of dendritic and tumor cells correlated with a decrease in the subpopulation of natural killer cells in the peripheral blood, which has prognostic value.
Helena Haque Chowdhury, Simon Hawlina, Mateja Gabrijel, Saša Trkov Bobnar, Marko Kreft, Gordan Lenart, Marko Cukjati, Andreja Nataša Kopitar, Nataša Kejžar, Alojz Ihan, Luka Ležaič, Marko Grmek, Andrej Kmetec, Matjaž Jeras, Robert Zorec (2021) Survival of castration-resistant prostate cancer patients treated with dendritic-tumor cell hybridomas is negatively correlated with changes in peripheral blood CD56 bright CD16 - natural killer cells. Clin Transl Med, 2021 Aug; 11(8): e505. doi: 10.1002/ctm2.505.
Since 2009, new legislation in the field of biological medicines for advanced therapy medicinal products (ATMPs) has been in force in the EU, consisting of tissue-engineered products, gene therapies and cell-based therapies. The first cell-based medicine to treat castration-resistant prostate cancer was approved in the United States in 2010 (sipuleucel-T), but despite the relative success of treatment, patients experienced quite a few serious adverse events.
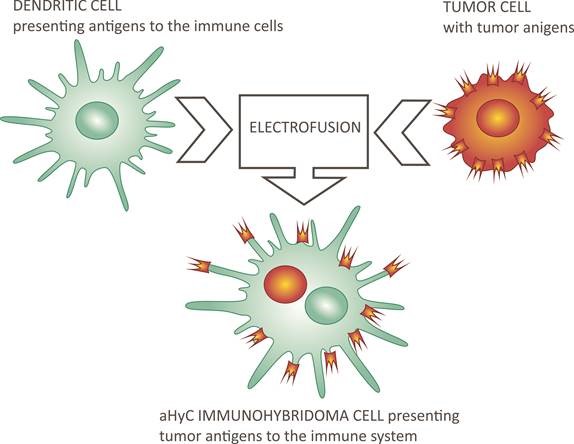
In order to reduce the latter, the the research and clinical group in Slovenia developed a fully autologous cell-based vaccine by preparing dendritic, antigen presenting cells from the patient's leukocytes, and combining them with the patient's own (autologous) cancer cells to form immunohybridoma (aHyC) cells by electrofusion, which the patient received intradermally. In such a completely personalized way, the process of presenting all, even unknown antigens of the cancer cell to the patient's immune system, starts in the body, since the cancer has an individual development of mutations in each patient. The results, published in the prestigious journal Clinical and Translational Medicine, show that no serious side effects have been reported in patients and that the efficacy of aHyC immunohybridoma therapy, as measured by patient survival, is robustly correlated with a decrease in peripheral blood NK leukocyte subpopulation. This is of particular value for monitoring disease progression. In addition, it opens up the possibility that the new technology for the preparation of aHyC immunohybridomas by electrofusion (HybriCure) can also be used to treat other forms of solid tumors.
It should be noted that the legislation in the field of ATMPs allows treatment under the so-called hospital exemption for medicines prepared on a non-routine basis (prepared for each patient individually) as there is only a few hours of shelf life before the immunohybridomas are administered into the patient. Therefore, the laboratory preparing such a cell vaccine must be close to the hospital / clinic where it is administered.